In the field of biomimetics, or bio-inspired engineering, nature’s designs form the basis of engineering projects. At the University of Arkansas College of Engineering, researchers in several different fields are looking to the natural world for ideas and tools that humans can use.
Russell Deaton, professor of computer science and computer engineering, agrees that nature’s designs make a good model for engineers. “Computer science has stolen from biology for years. Living things embody intelligence. At this molecular scale, nature is doing computations. Biology is all about information processing.”
A Lesson on Light
Roper is walking a group of students from Bell Engineering Center to his lab in the Nanoscale Materials Science and Engineering building across the street, and he begins their lesson by pointing to the sky.“Why do we see blue and not yellow or red?” he asks. “Molecules in the air scatter particular frequency of light from the solar spectrum relative to all the other frequencies. The molecules don’t interrupt or scatter red, yellow or orange. But they do scatter blue light.”Roper finds it interesting interesting is that certain organisms have evolved complicated optical surfaces to capture and diffract light. Imagine you are holding a peacock feather, for example. As you turn the feather, the colors shift: blue, purple, green. These feathers, along with the wings of certain moths and butterflies, have special surfaces that cause light waves to interfere with each other, making the shifting colors we call irridescence.Why, he wonders, would nature go to all that trouble? And what could engineers learn from these organisms?
In the lab, the students peer through a powerful microscope at irridescence on the nanoscale. Starting with a polymer called poly(dimethylsiloxane), Roper and his students have formed nano-sized features on one surface of the material. These features alter the local electromagnetic field of a thin layer of material, causing different colors to appear depending on the angle of the light and dimensions of the features. In some ways this surface mimics the texture of an irridescent butterfly wing, but Roper’s research goal is to develop a tool that does much more than make pretty colors.
Prisms That Catch Tumors
Roper wants to use this prism effect to identify biomarkers for malignant tumors.
Roper is studying the way different biomarkers, strands of DNA or amino acids that are particular to cancer cells, interact with light. His goal is to be able to make a tiny device that could be used externally to monitor the bloodstream for these biomarkers. The device would use gold nanoparticles patterned like peacock feathers to diffract the incident light. As this diffracted light hits different proteins or DNA in the blood, it would produce characteristic patterns which could be analyzed to identify a tell-tale pattern produced by biomarkers for disease.
“If you can harness the ability to capture and diffract light,” Roper explained, “you can use nanoscale structures in a way that humans can interact with directly in complex biological systems, instead of using more expensive, complicated equipment.” Diagnostic tools that use light patterns would be more sensitive to subtle indicators of disease and easier to indentfy and interpret—different colors and patterns could indicate the presence of particular disease markers without requiring expensive or toxic labels.
“It could detect metastatizing cancers that aren’t detectable by other means,” said Roper, “identifying if it’s there and where it’s located, like a policeman with a radar gun.”
Algae That Make Fuel
Recently, Roper also started studying diatoms, a type of algae made up of single-celled organisms. He suspects that diatoms may capture and focus light through optical structures, the way a lens in a microscope does.
While most plants turn sunlight into energy, diatoms do this differently. “Photosynthesis takes light and uses the light in an electron transport chain,” said Roper. In this process, the plants use chlorophyll to turn light into fuel. “But diatoms don’t rely primarily on chlorophyll. They utilize a different region of the electromagnetic spectrum—the way they access light is different.” Because diatoms are able to thrive underwater or under the soil, where exposure to direct light is reduced, Roper suspects their method of harvesting energy from the sun is more efficient than that of other plants at wavelengths that have practical uses.
By studying their optical structures, Roper hopes to find new, more efficient ways to produce and store energy from light.
“These are exquisite, patterned, crystalline structures made by a single cell,” he said. “If we could figure out how to do make these structures as elegantly as diatoms do, we could reproduce them in large numbers to greatly increase our ability to harvest light.” Then these structures could be used to produce chemical energy, the same way that diatoms produce the chemicals they use as food. “Algae can make liquid chemical energy that has higher fuel value and is more transportable than gaseous fuels,” said Roper. “Harnessing light absorption methods of algae could provide clean, renewable transportation fuels.”
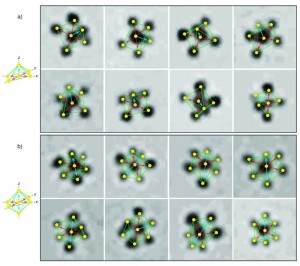
Using DNA blueprints, these nano-sized nBLOCKs have arranged themselves into different shapes. Lines superimposed over the TEM images illustrate the geometric trends. Adapted from Kim et al. (2011)
Nanostructures That Build Themselves
DNA contains nature’s source code. Using four different molecules—adenine, thymine, guanine and cytosine—DNA creates the instructions that are essential to life. Jin-Woo Kim and Russell Deaton are hoping to use this system to create their own instructions, a set of codes that arrange nanoparticles into structures.
Kim and Deaton are affixing gold nanoparticles to strands of DNA. Then, they can allow the DNA to use its code, attaching adenine to thymine and guanine to cytosine, to self-assemble the particles into larger structures. What kinds of structures? The possibilities are endless. “It could have many different applications, and we’re investigating what we can make,” said Kim. “We have a Lego block.”
Kim and Deaton have demonstrated that their Lego blocks, or nBLOCKS as they call them, exhibit chemical stability and water solubility, and that they remain stable at temperatures up to 100 degrees Celcius and down to 4 degrees Celcius. Using these blocks, researchers could make customized materials for biomedical applications, for example. Kim explained that they could enhance the optical response of the nanoparticles and use that for diagnostic purposes, or to localize radiation for cancer treatment. But the first step is perfecting the building blocks.
In the lab, Kim is working on the process of binding the nanoparticles to the DNA with his post-doc, Jeong-Hwan Kim. First, he mixes the nanoparticles and the DNA in a small test tube, using plastic silica gel to keep the DNA from clumping. Each nanoparticle binds to one DNA strand, and then Kim removes the DNA from the silica gel and repeats the process, binding another strand of DNA to each particle. In this way, Kim can bind up to six DNA strands to each particle, forming links on the x, y and z axes.
In order to be sure that the correct number of nanoparticles are bound to the DNA strands, Kim performs several tests. He measures the response of the particles to light with an ultraviolet-visible spectrometer and separates the particles according to size with gel electrophoresis. With these two tests, he can tell if both gold and DNA are present in the samples, and he can tell which particles have the most strands of DNA attached.
Finally, once the other two tests have verified that the particles have the correct number of strands attached, Kim examines the nBLOCKS under a transmission electron microscope and an atomic force microscope to measure the locations and angles between DNA strands on the particle. Getting DNA geometry correct is an important part of the process—if the geometry is not exact, the three dimensional structures that result will be lopsided.
Designing with DNA
The next step of the research will be putting these blocks together to form shapes, and the shapes that result will be determined by the code in the DNA. If the codes are correct, the strands of DNA will find each other and self-assemble into the correct shapes, taking the particles along with them.
It’s Deaton’s job to use computational modeling to figure out the code behind the shapes they want to make. “It really boils down to how many DNA sequences we have to use,” he said. “If we use a different sequence for every connection we want to make, then we have a lot of control.”
However, even with strands that are 20 bases long, the researchers would run out of unique sequences eventually, and this would limit the size and complexity of the structures they could make. In addition, the researchers are limited by cost, because they must pay for each unique strand of DNA.
The key is figuring out how to build structures in which some of the links are duplicate sequences, while ensuring that these copies won’t interfere with the overall design. “That’s where the complexity really enters into the design part,” said Deaton. “We’d like to use as few DNA sequences as possible, but deciding on what that number actually is, is a hard problem.”
Perfecting this system will be a long process, but Kim points out that nature can teach engineers lessons about patience, as well. “That’s another thing we can learn from nature. Everything doesn’t happen at once; things happen stepwise and then merge together.”
Much More to Learn
“The thing about biology,” said Deaton, “is that we don’t know everything. It’s not a quantified thing—there are a lot of missing components.”
Roper agrees. He explained that biotechnology has made some incredible advances, especially in the area of pharmaceuticals, “but not in ways that were originally envisioned…The deeper you get into it,” he said, “the more you find layers on layers on layers of regulatory balance in every organism,” he said. “We’re on the tip of the iceberg. If we were aware of all the ways we interact with our environment, it would boggle our minds.”
Roper’s research takes place with help from current and former graduate students: Wonmi Ahn, Phillip Blake, Drew Dejarnette, Gyoung gug Jang, Braden Harbin, and Laura Velasco. It is supported by Shi Chi Liu of the Sensors and Sensing Systems Program in the Division of Civil, Mechanical, and Manufacturing Innovation at the National Science Foundation, the Ralph E. Martin Department of Chemical Engineering, the Institute of Nanoscale Material Science and Engineering, the microEP graduate program, the University of Arkansas Foundation, and the Walton Family Foundation. Part of the algae project was completed with funds from Statoil, with co-PIs Bob Beitle and Jamie Hestekin. Deaton and Kim’s research is supported by the National Science Foundation, the University of Arkansas Division of Agriculture and the Arkansas Biosciences Institute. Rainbow image courtesy of jmci, istock.com Algae image courtesy of Carolina Biological Supply Company nBLOCK image courtesy of Kim, J.-W., Kim, J.-H. & Deaton, R. DNA-linked nanoparticle building blocks for programmable matter. Angewandte Chemie International Edition 50, 9185-9190 (2011)